A Solvent Can Be Described by Which of the Following
Contains more than the maximum amount of solute. A solvent is typically an organic liquid.

Lesson Explainer Mixtures Nagwa
___________ is a good compromise solvent.
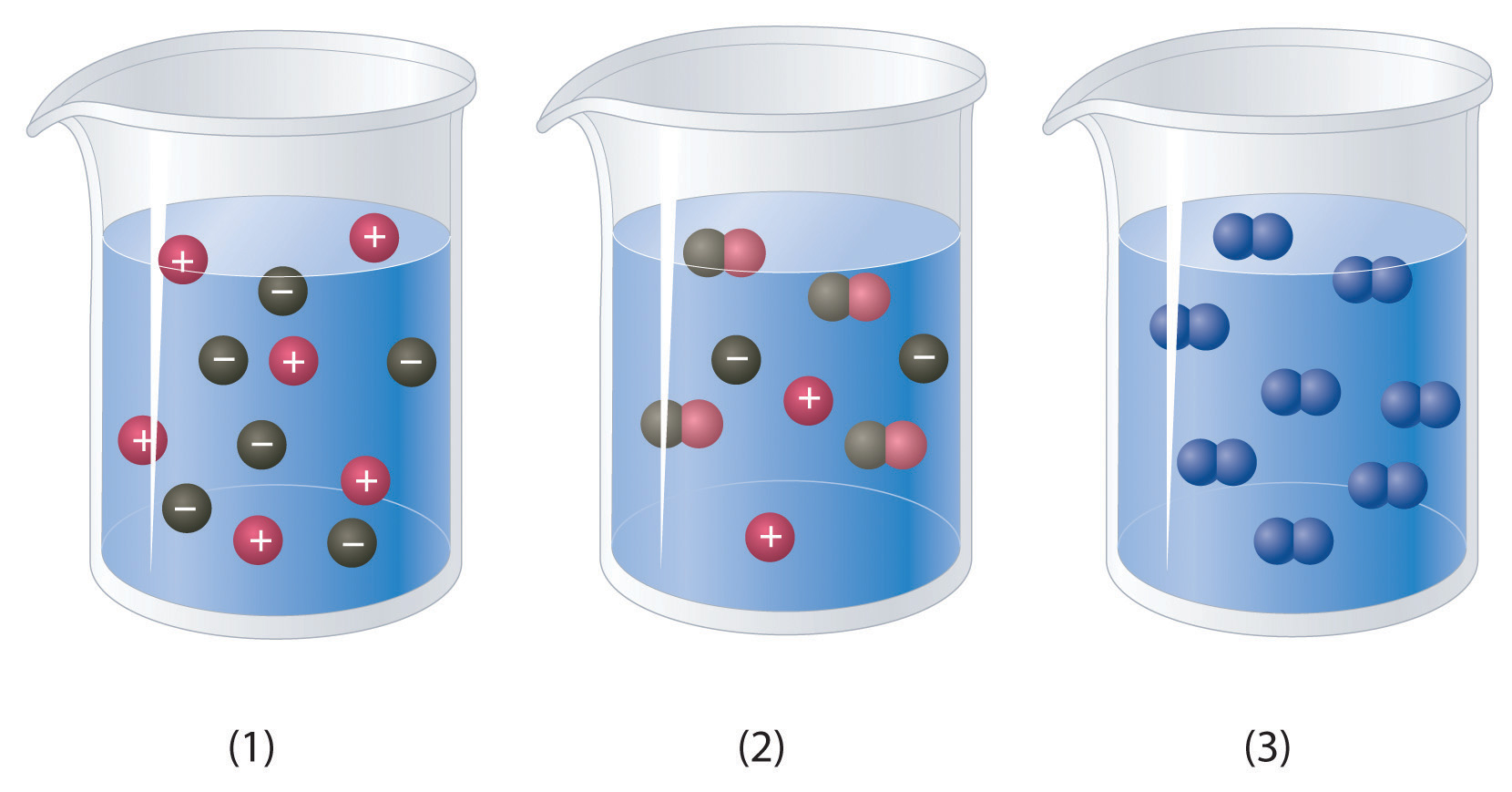
. Concentration can be described qualitatively using the words concentrated or dilute. Water is an ideal solvent in cells because it. Solution A mixture of one or more solutes dissolved in a solvent.
The solubilityof a solute is the amount of solute that will dissolve in a given amount of solvent to produce a saturated solution. However the extraction yield and the antioxidant activity of the extracts can be vary with the type of solvent. Following the reaction an extraction can be employed to pull the desired.
Some of the most common solvents include water ethanol and methanol. Surface tension can be described as a property of a liquids surface that allows it to resist an external force due to their cohesive nature of the solvent molecules. Solvent The substance in which a solute dissolves.
In the deformation of polymers the bonds between the oxygen and hydrogen that connected the monomers are broken. The following rules can be used to decide which component of asolution is the solute and which is the solvent. According to mathematical models F c is dependent on changes on several parameters including radical.
Suppose a solution is described as concentrated. A solution that contains the maximum amount of solute that is capable of being dissolved. Now solving for n.
A concentrated solution contains a large amount of solute A dilute solution contains a small amount of solute We also can express concentration quantitatively. Threshold of 50 psi is present on the solvent kegs described below. Maximum amount of solute dissolved in solvent at specific temp.
The oxygen end of water molecules is attracted toward Ca 2 ions. P 2 pressure on product side subscript 2 product or. What is the name for the process of disassociating the monomers from a polymer using water as a solvent.
Thus when resistance is low faster and evenly spreading is observed. Where N w solvent water flux kgm 2 s. B A solution can have only one solute.
Specific amount of solvent or solutions. The concentration of a solution can be described in several ways. Following the reaction you added water to the reaction flask to quench certain reactive reagents.
Contains less than the maximum amount of solute that a solvent can hold at specific temperature saturated. The exclusion is very specific and only includes items that meet the definition a solvent-contaminated wipe. A waste determination in accordance with 40 CFR 26211 must be made on wipes that are contaminated with waste other than the ones described in the definition for solvent-contaminated wipes.
The random movement of molecules in a solvent causes which of the following in a cube of solid solute. A solution in which more solute will dissolve or. In which way do we calculate the moles of a solute divided by the kilograms of solvent.
A solution that contains more than the maximum amount of solute that is capable of being dissolved. Here F c is defined as the ratio of the rate constant for cage recombination k c to the sum of the rate constants for all cage processes. A can be dissolved by the solute B has a high concentration of solutes C usually a sugar or protein D present in a greater concentration that solutes Which of the following phrases best describes a solvent.
P w solvent-membrane permeability kg solventmatms. Low surface tension allows a solvent to spread uniformly and rapidly on a contact surface resulting in excellent wetting. A w solvent permeability constant kg solventm 2 atms.
The cage effect can be quantitatively described as the cage recombination efficiency F c where. A solvent is widely used in consumer products. Eventually the molecules of solute become evenly distributed in throughout the solvent.
In conclusion a solvent is a substance present in a greater. A solvent can be solid liquid or gas. There are two control valves one is an argon inlet and the other is the solvent outlet under normal working conditions.
The solvent kegs can hold up to 50 liters of solvent. The solvent has the ability to dissolve other substances present in the solution that are referred to as solutes. P 1 pressure on feed side subscript 1 feed or upstream side of the membrane atm.
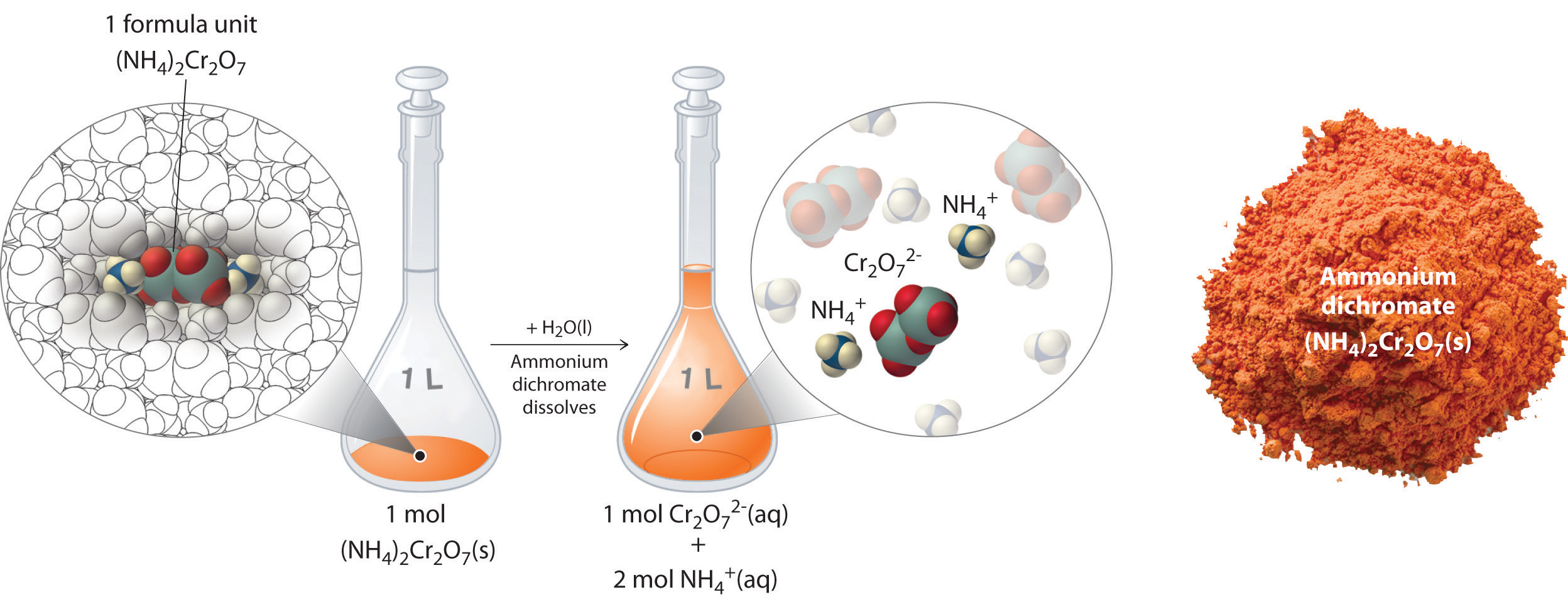
Hydration is a special case of solvation in which the solvent is water. The solute must be the same physical state as the solvent. A solvent is a molecule that has the ability to dissolve other molecules known as solutes.
A substance in which solute is dissolved and forms solution is a solvent. L m membrane thickness m. Solids liquids and gases.
A I II and III b I and II c III d I e II. THF and water are miscible so a homogeneous solution results. Generally solvent is a liquid but it can also be a solid a gas or a supercritical fluid.
Which of the following statements can be concluded. In such solvent similarly as in water the pH of neutrality corresponds to. A solution in which no more solute will dissolve.
A solvent is widely used in consumer products. On the basis of polarity there are two types of solvents they are polar and non-polar. As per the solvent the polarity of the solvent is an important factor.
We can do this by using molarity. A solvent is a substance usually a liquid that dissolves another substances in order to form a solution. A solution that contains less than the maximum amount of solute that is capable of being dissolved.
C A solution can have many solutes. N 125 à 125 g of X in the ether layer. Contains the maximum amount of solute that a solvent can hold at specific temperature supersaturated.
The extent of the pH scale which in aqueous media can be described as 14 units depends on the autoprotolysis constant of the amphiprotic solvent so that the equivalent range eg in methanol equals 167 units in sulfuric acid 29 units in acetic acid 145 units. The molecules of the solvent work to put the solute molecules apart. The term Solvents refers to a class of chemical compounds described by function the term derives from Latin meaning roughly to loosen.
1aA solvent can be described by which of the following. This can be described as. ΔPhydrostatic pressure difference atm.
Which of the following molecules can participate in dipole-dipole interactions. There are three states of matter. The hydrogen end of water molecules is attracted toward Cl- ions.
In chemistry solvents which are generally in liquid form are used to dissolve suspend or extract other materials usually without chemically changing either the solvents or the other materials.

Lesson Explainer Polar And Nonpolar Solvents Nagwa
Comments
Post a Comment